A problem often encountered by biologists centers around defining appropriate protein and drug (factor) concentrations in a mixture of signaling factors to realize a desired biological outcome. For example, this outcome may be realized in the production of a particular cell-type to be used either as a tool for discovery or a therapeutic. Current high-throughput robotic screening technologies allow for single factors at a single concentration to be dispensed into a single well. Unfortunately, this strategy limits the experimental scope, as it is expensive in both time and capital. We are defining a strategy, “combinatorial factor screening”, as a method to overcome these obstacles. This strategy utilizes a standard liquid handling platform capitalizing on the knowledge of how to control the pipetting software through an in-house software model. This strategy may be applied in both fields of biology and chemistry in both academia and pharmaceutical industries.
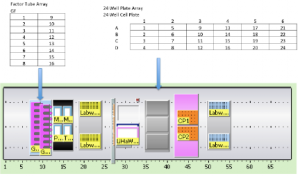
An optimization model has been developed for this project by Michael Ferris. Input to the model is a list of experiments e, each of which involve different amounts of input factors f . Typically there are over 1000 experiments and around 10 factors.
The aim of the model is to assign each experiment e to a specific well on a standard multi-well tissue culture plate in order to minimize the total time needed to complete the set up of all the experiments.